Dess-Martin Oxidation
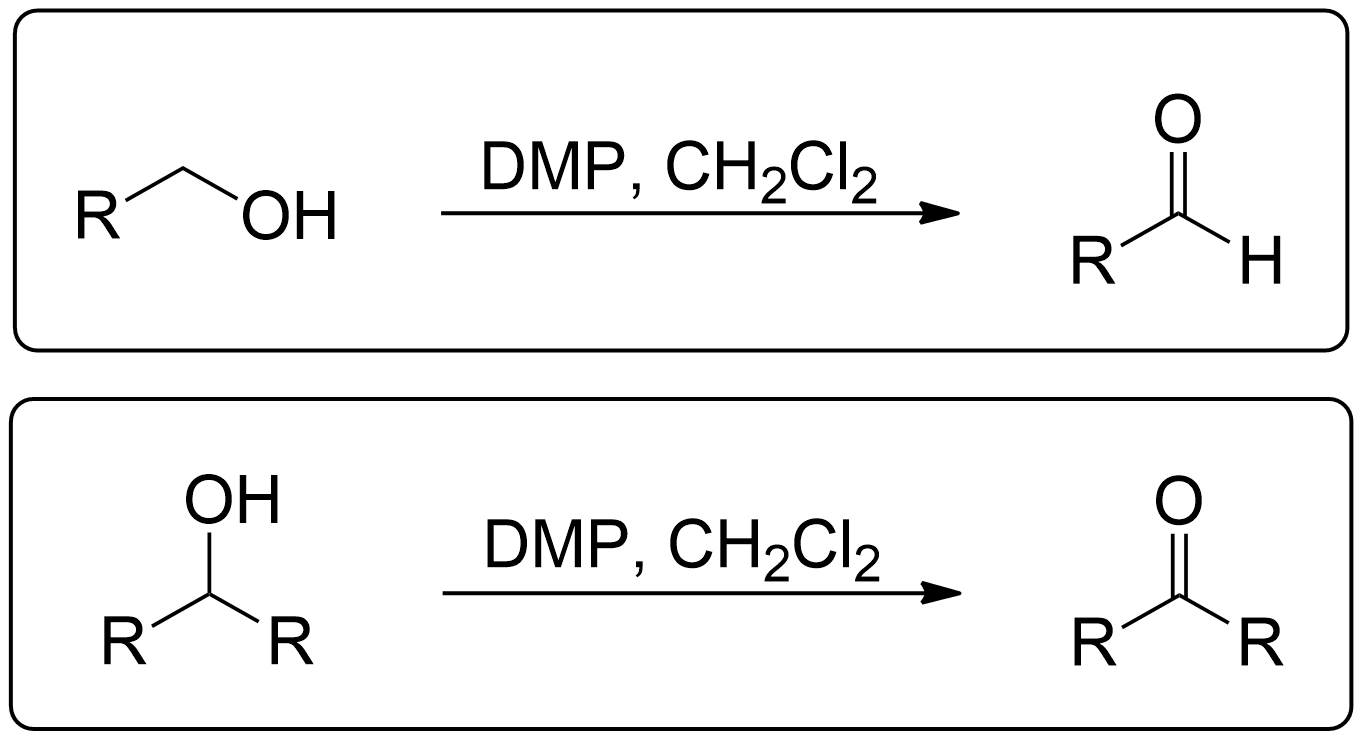
General Scheme of Dess Martin Oxidation
The Dess-Martin oxidation is a method used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones using the Dess-Martin periodinane (DMP). This reagent is known for its mild conditions, high selectivity, and efficiency, making it a valuable tool in organic synthesis.1
Finding the Correct Product for a 1° Alcohol

Propose a Mechanism for this Reaction.
We must find out how the product was formed and the steps to form it.
(+)-Cortistatin A, a potent inhibitor of endothelial cell proliferation, was synthesized using enantiomerically pure Hajos-Parrish ketone as the starting material. The synthesis involved converting the Hajos-Parrish ketone to a known enone and then to a silyloxydiene intermediate.2
A crucial step in this multi-step process, as depicted in the image, was the oxidation of a primary alcohol intermediate to an aldehyde using Dess-Martin periodinane (DMP) (1.2 equiv) in dichloromethane (DCM) at room temperature for 1 hour. This transformation played a vital role in advancing the synthesis.2
The resulting aldehyde intermediate was then further utilized in subsequent steps towards the total synthesis of (+)-Cortistatin A.
Identify the Reagents

Often times, Dess Martin Oxidation is performed with these 2 reagents mainly
- Dess-Martin Periodinane (DMP)
- DCM as a solvent
Identify the Key Features of the Compound
Alcohol Type

These are the 3 main types of alcohols: Primary, Secondary and Tertiary.
- Secondary alcohols can go through Dess-Martin Oxidation to become a Ketone.
- Primary alcohols can go through Dess-Martin Oxidation to become an Aldehyde.
By Identifying the Alcohol Type, you now know the product to expect.
Side Chain and Product

Always Identify the Side Chain vs. Alcohol
Identify Colored Side Chains: Recognize the colored side chains in the diagrams. These colors are placeholders represent R groups on both sides of the Ketone or a singular R group on one side of the Aldehyde that remain unchanged during the reaction.
Understand Their Role: The colored side chains help track which parts of the molecule on both sides of the Ketone or a singular R group on one side of the Aldehyde are not involved in the reaction. They stay constant, ensuring clarity in following the chemical changes.
Focus on the Reaction Center: Concentrate on the alcohol group, which undergoes oxidation to become a ketone or aldehyde. The side chains, highlighted in colors, will guide you in visualizing the molecular structure before and after the reaction.
Reassign Side Chains: Once the reaction is complete, use the colored side chains to reattach them conceptually to the new ketone or aldehyde, demonstrating the selective nature of this oxidation without altering the side chains’ position or identity.
Remember, the colored side chains are visual tools to help you understand and follow the molecular changes during the Dess-Martin oxidation.
- Dess-Martin Oxidation is always carried out in mild [O] conditions.
- Side chain (R) remains the same the entire reaction.
- The 1° (Primary) alcohol is the section of the molecule that undergoes change.
Disclaimer Warning for Writing Products

Aldehydes and Ketones may be presented differently in different questions but in the end they are all the same
How do I perform the Mechanism?
Formation of Reactive Intermediate

Alcohol group (-OH) performs a nucleophilic attack on the iodine center of the DMP molecule. This interaction results in the formation of a complex where the alcohol is temporarily bonded to the iodine.
As a result of this reaction intermediate forming, an OAc group is expelled from the compound.
Charge Stabilization using a Carboxylate Ion

The Newly formed Intermediate is stabilized using the expelled -OAc group (Carboxylate Ion).
Acetic Acid Recovery and Product Formation

1 mole of Acetic Acid is recovered and the intermediate undergoes proton transfer initiated by another carboxylate ion to form the Aldehyde product
Overall Products

- Aldehyde is formed as a result of a primary alcohol
- Iodinane is one of the by-products post-oxidation
- 2 moles of Acetic Acid is recovered post-oxidation
Reconstructing the Overall Product

Reconstruction of the Target Intermediate
Mechanism for 2° Alcohol

Propose a Mechanism for this Reaction.
Ingenol, the parent compound of several naturally occurring ingenanes with varied peripheral functionalities, was synthesized through a series of steps. These ingenanes display a range of interesting biological profiles, from tumor-promoting to anti-leukemic and anti-HIV activities.3
One key step in the synthesis involved the oxidation of a secondary alcohol intermediate to an ketone using Dess-Martin periodinane (DMP) in dichloromethane (DCM). This transformation was achieved with a 74% yield and was crucial in advancing the synthesis.3
The resulting ketone intermediate was further utilized in the total synthesis of Ingenol to form additional intermediates.
Identify the Side Chains and Alcohol Type

The side chains are colored to denote unchanging R groups. Observe how the alcohol group is selectively oxidized to form a ketone or aldehyde. The colors help you visually track and reposition these side chains after the reaction, showing the specificity of the oxidation.
Perform the Mechanism: Form Intermediate

The steps are the same as the mechanism of a primary alcohol, just add an extra side chain group in your mechanism
Carboxylate Ion stabilizes Intermediate

Intermediate is stabilized using the expelled -OAc group (Carboxylate Ion)
Acetic Acid Recovery and Product Formation

Acetic Acid is recovered and the intermediate undergoes proton transfer initiated by another carboxylate ion to form a Ketone
Overall Products

Same Products as the Primary Mechanism except instead of an Aldehyde Product, its a Ketone
Reconstructing the Overall Product

Target Intermediate Reconstruction
Sample Problems
Predict the Product

Guess the Product.
Clostrubin, a potent antibiotic against methicillin- and vancomycin-resistant bacteria, was synthesized through a series of steps, including the desilylation of a silylated intermediate during acid workup, resulting in the formation of a secondary alcohol intermediate.4
Reveal the Answer

This compound had a secondary alcohol that was oxidized to a Ketone
This alcohol intermediate, as depicted in the image, was then oxidized to a ketone using Dess-Martin periodinane (DMP) in dichloromethane (DCM).4
This transformation was achieved with an 83% yield and preserved the sulfur-containing functionalities.4
The resulting ketone intermediate was further utilized in the total synthesis of clostrubin to form additional intermediates.
Mechanism Problems

Propose a Mechanism for this Reaction.
(+)-Preussin, a potent antifungal agent, is synthesized using protected L-N-benzoyl phenylalaninol as a starting material.5
This alcohol intermediate underwent oxidation using Dess-Martin periodinane (DMP) in dichloromethane (DCM) to yield the corresponding aldehyde without racemization. This transformation was achieved with a specific yield that was unreported in the paper and was a crucial step towards advancing the synthesis.5
The resulting aldehyde intermediate was then further utilized in subsequent steps towards the total synthesis of (+)-Preussin.
Reveal the Answer

Colored side chains in these diagrams represent constant R groups. Focus on the central alcohol, which transforms into a ketone or aldehyde. Use the colored chains to track and restore these groups post-reaction, highlighting the selective oxidation process.

Form the Overall Product

This should form the expected Ketone product as a result of oxidation of the primary alcohol on this compound.
Authors Notes / FAQs
What citation style do you use for your references?
How do I access the papers you referenced for your examples?
Why are there no step numbers included in the reaction diagrams?
Our approach prioritizes simplicity and clarity to enhance effective learning, particularly for beginners. Although scientific publications often include reaction step numbers, we have chosen not to include them to maintain clarity and avoid confusing readers, especially when presenting yields alongside the reaction. This approach aligns with the practices in textbooks and other educational resources where reaction step numbers are typically not displayed.
By focusing on the essential details—such as reagents, conditions, and yields—we prevent information overload and ensure that the key information is easily accessible. Detailed descriptions of the reaction steps and additional context are provided in the accompanying text, offering a comprehensive understanding without cluttering the diagrams.
Including reaction step numbers might lead to questions about the entire synthesis pathway, which is not our intention. Our goal is to highlight specific reactions occurring in papers and explore them in detail, rather than presenting the entire synthesis process. This focused approach allows readers to quickly grasp the main points of each reaction while providing the option to delve deeper into the details as needed.
Our objective is to create a resource that is both informative and user-friendly, catering to learners at all levels by emphasizing clarity and relevance in presenting chemical reactions.
Why is the yield percentage included in the diagram?
How do you handle complex multi-step reactions in the papers?
Simply put, we isolate the specific step in the synthesis where the Dess-Martin oxidation takes place, cite it, and illustrate the mechanism to reach the product as described in the paper. We do not provide the entire synthesis of the paper but rather focus on the key step that showcases the specific article topic’s reaction (in our case, Dess-Martin oxidation). This approach ensures clarity and relevance, allowing readers to focus on the specific transformation of interest without being overwhelmed by the entire synthetic pathway.
By focusing on the most relevant aspects of the reactions, we aim to provide a clear and concise educational resource that caters to learners at all levels. We appreciate feedback and are open to constructive suggestions on how to enhance the clarity and educational value of our content.
References

1. Dess, D. B.; Martin, J. C. Readily Accessible 12-I-51 Oxidant for the Conversion of Primary and Secondary Alcohols to Aldehydes and Ketones. J. Org. Chem. 1983, 48, 4155–4156. DOI: 10.1021/jo00356a052. ↩

2. Lee, H. M.; Nieto-Oberhuber, C.; Shair, M. D. Enantioselective synthesis of (+)-cortistatin A, a potent and selective inhibitor of endothelial cell proliferation. J. Am. Chem. Soc. 2008, 130 (50), 16864–16866. DOI: 10.1021/ja8071918. ↩

3. Nickel, A.; Maruyama, T.; Tang, H.; Murphy, P. D.; Greene, B.; Yusuff, N.; Wood, J. L. Total synthesis of ingenol. J. Am. Chem. Soc. 2004, 126 (50), 16300–16301. DOI: 10.1021/ja044123l. ↩

4. Yang, M.; Li, J.; Li, A. Total synthesis of clostrubin. Nat. Commun. 2015, 6, 6445. DOI: 10.1038/ncomms7445. ↩

5. Lee, K.-Y.; Kim, Y.-H.; Oh, C.-Y.; Ham, W.-H. Facile and efficient total synthesis of (+)-preussin. Org. Lett. 2000, 2 (25), 4041–4042. DOI: 10.1021/ol000289p. ↩