PCC Oxidation
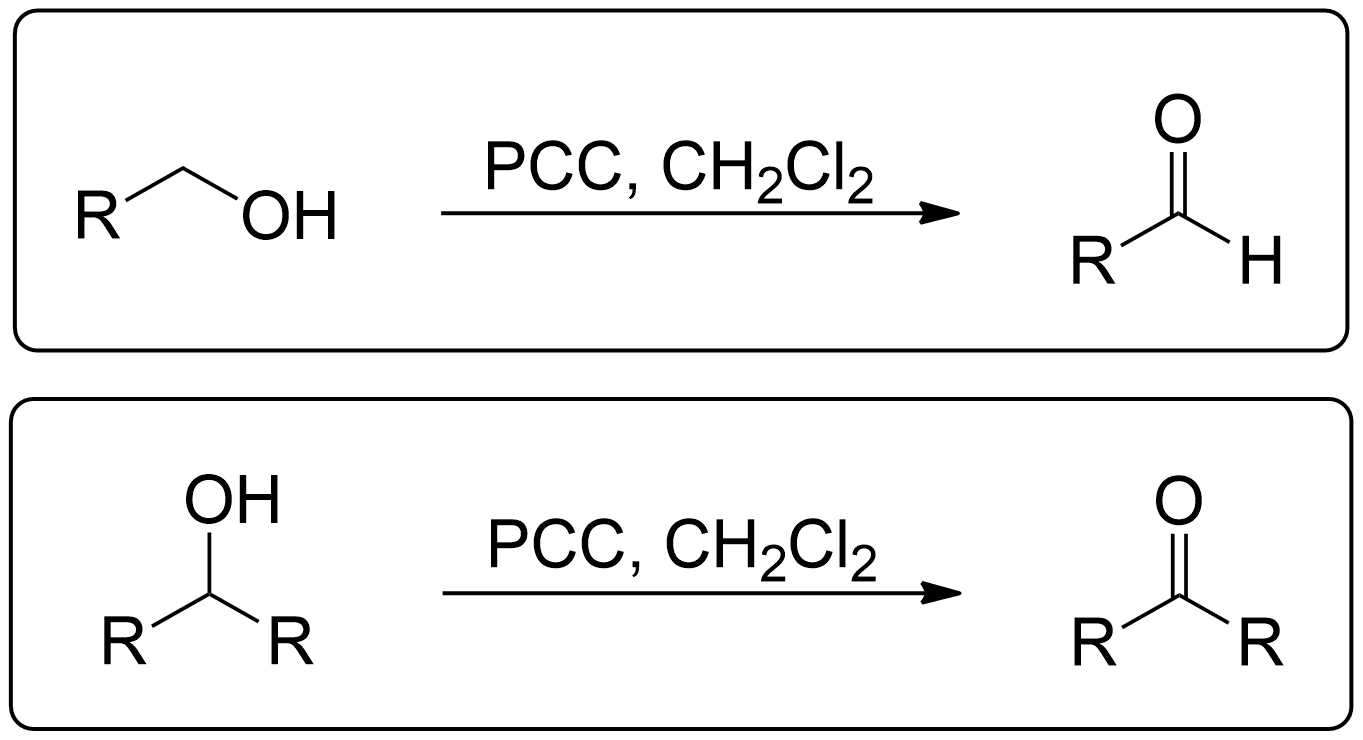
General Scheme of PCC Oxidation
Finding the Correct Product for a 1° Alcohol

Assume you've been given this question: What do you do?
Identify the Reagents

PCC and DCM - Good for Mild Oxidation
PCC Oxidation uses PCC (Pyridinium Chlorochromate) which is a salt soluble in halogenated organic solvents such as DCM which allows anhydrous reaction (Mild).
Identify the Key Features of the Compound
Alcohol Type

These are the 3 main types of alcohols: Primary, Secondary and Tertiary.
- Secondary alcohols can go through PCC Oxidation to become a Ketone.
- Primary alcohols can go through PCC Oxidation to become an Aldehyde.
By Identifying the Alcohol Type, you now know the product to expect.
Side Chain and Product

1° (Primary) Alcohols always yield an Aldehyde
Identify Colored Side Chains: Recognize the colored side chains in the diagrams. These colors are placeholders representing R groups on both sides of the Ketone or a singular R group on one side of the Aldehyde that remain unchanged during the reaction.
Understand Their Role: The colored side chains help track which parts of the molecule on both sides of the Ketone or a singular R group on one side of the Aldehyde are not involved in the reaction. They stay constant, ensuring clarity in following the chemical changes.
Focus on the Reaction Center: Concentrate on the alcohol group, which undergoes oxidation to become a ketone or aldehyde. The side chains, highlighted in colors, will guide you in visualizing the molecular structure before and after the reaction.
Reassign Side Chains: Once the reaction is complete, use the colored side chains to reattach them conceptually to the new ketone or aldehyde, demonstrating the selective nature of this oxidation without altering the side chains’ position or identity.
Remember, the colored side chains are visual tools to help you understand and follow the molecular changes during the Corey-Schmidt Oxidation.
- PCC Oxidation is always carried out in mild [O] conditions.
- Side chain (R) remains the same the entire reaction.
- The 1° (Primary) alcohol is the section of the molecule that undergoes change.
Disclaimer Warning for Writing Products

Aldehydes and Ketones may be presented differently in different questions but in the end they are all the same
How do I perform the Mechanism?
Nucleophillic Addition to PCC

Alcohol group (-OH) performs a nucleophilic addition on the Chromium center of the PCC molecule.
Interaction between Alcohol and PCC results in the formation of a complex where the alcohol is temporarily bonded to Chromium. The intermediate is subsequently stabilized for further proton transfer.
Charge Stabilization via Proton Transfer

Newly Formed Intermediate violates the Octet Rule and is stabilized
Removal of Chloride through SN2

Chloride is expelled from the Intermediate via SN2 reaction
Proton Transfer to yield Final Products

Expelled Chloride Ion initiates proton transfer to yield the final products
Final Products Overview

Chromous Acid and Pyridinium chloride are the Byproducts
Build the Overall Product

Rebuilding Process of the Target Compound
The side chains are colored to denote unchanging R groups. Observe how the alcohol group is selectively oxidized to form the aldehyde. The colors help you visually track and reposition these side chains after the reaction, showing the specificity of the oxidation.
Mechanism for 2° Alcohol

Assume you've been given this question: What do you do?
Identify the Side Chains and Alcohol Type

The Identification Steps are the same
The side chains are colored to denote unchanging R groups. Observe how the alcohol group is selectively oxidized to form the ketone. The colors help you visually track and reposition these side chains after the reaction, showing the specificity of the oxidation.
Perform the Mechanism: Form the Intermediate

The steps are the same as the mechanism of a primary alcohol, just add an extra side chain group in your mechanism
Intermediate Stabilization

Stabilization of the Intermediate
SN2 Reaction for Chloride Removal

Expulsion of the Chloride
Proton Transfer to yield Final Products

Product Formation Step
Final Product Overview

All the products and By-products
Build the Final Product

All the products and By-products
Sample Problems
Predict the Product

Guess the Product
Reveal the Answer

This compound had a secondary alcohol that was oxidized to a Ketone
Mechanism Problems

Propose a Mechanism for the conversion of Lupeol to Lupenone
Reveal the Answer

Colored side chains in these diagrams represent constant R groups. Focus on the central alcohol, which transforms into a ketone or aldehyde. Use the colored chains to track and restore these groups post-reaction, highlighting the selective oxidation process.

Form the Final Product

This should form the expected Ketone product as a result of oxidation of the primary alcohol on this compound.
Links for Educators
We aim for our PCC Oxidation resources to empower educators and students to deepen their understanding of selective oxidation processes. Through detailed diagrams and streamlined explanations, we hope to simplify complex concepts, enhancing both teaching and learning in organic chemistry.