Swern Oxidation
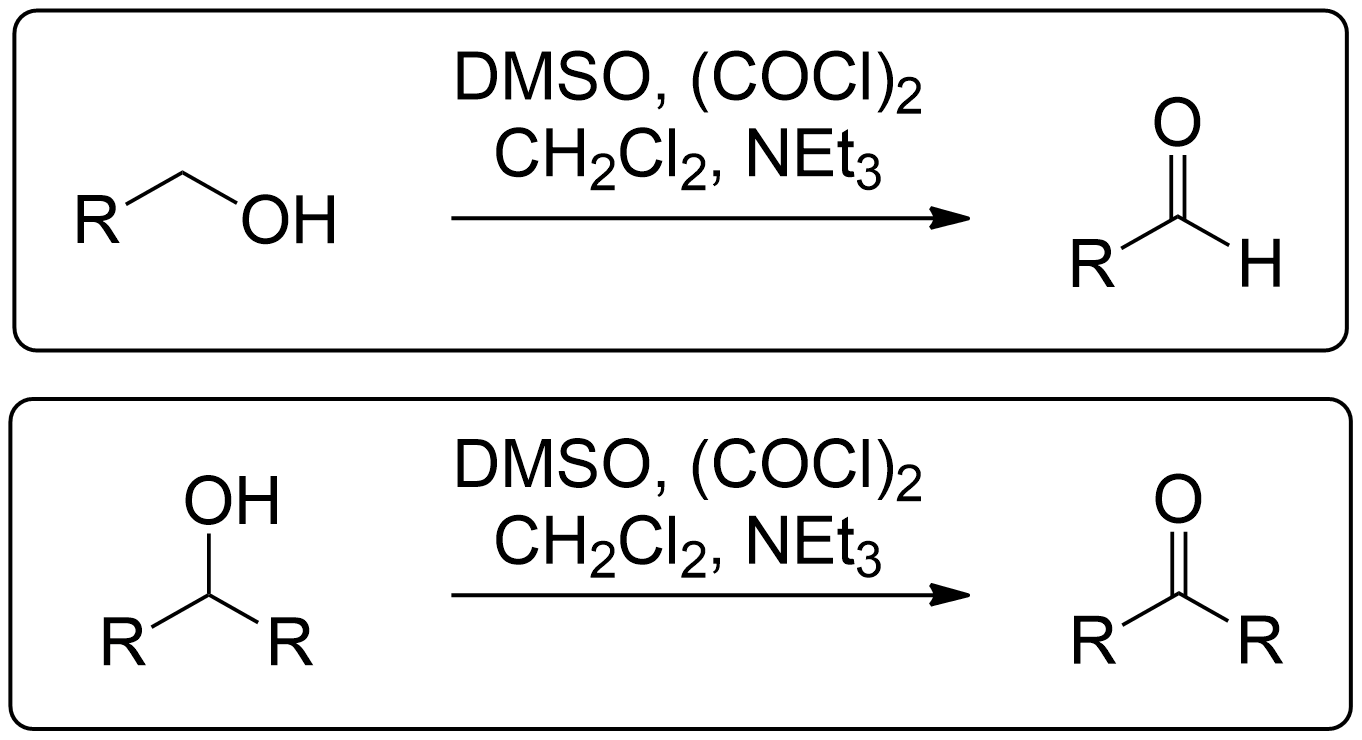
Reaction Overview of Swern Oxidation
Finding the Correct Product

Assume you've been given this question: What do you do?
Identify the Reagents

Often times, Swern Oxidation is performed with these 4 reagents or a combination of some of the reagents:
- DMSO
- Oxalyl Chloride
- Triethylamine (TEA)
- DCM as a solvent
The main reagents are DMSO and Oxalyl Chloride.
Identify the Key Features of the Compound
Alcohol Type

These are the 3 main types of alcohols: Primary, Secondary and Tertiary.
- Secondary alcohols can go through Swern Oxidation to become a Ketone.
- Primary alcohols can go through Swern Oxidation to become an Aldehyde.
By Identifying the Alcohol Type, you now know the product to expect.
Side Chain and Product

1° (Primary) Alcohols always yield an Aldehyde
Disclaimer Warning for Writing Products

Aldehydes and Ketones may be presented differently in different questions but in the end they are all the same
- Swern Oxidation is always carried out in mild [O] conditions.
- Side chain (R) remains the same the entire reaction.
- The 1° (Primary) alcohol is the section of the molecule that undergoes change.
How do I perform the Mechanism?
DMSO undergoes Resonance

Chlorosulfonium Ion Formation

DMSO Resonance Structure performs Nucleophilic Attack, Chloride Ion acts as a Leaving Group
Chlorodimethyl Sulfonium Ion and Byproduct Formation

The Chloride Ion, previously the Leaving Group, performs a Nucleophilic Attack
The nucleophilic attack initiates proton transfer within the Chlorosulfonium Ion to form Chlorodimethyl Sulfonium Ion its Byproduct.
SN2 Substitution and Stabilization

The Alcohol and the Chlorodimethyl Sulfonium Ion to form a Alkoxysulfonium Ion, the Oxygen in the Alcohol is violating the octet rule and has a Positive Charge.
The Octet rule is being violated in the Alkoxysulfonium Ion. Therefore, Triethylamine (TEA) is being used to neutralize the charges and stabilize the molecule for further transformation.
Product Formation

- TEA reacts with the Alkoxysulfonium Ion and proton transfer occurs to prepare the Ion for product formation
- Intramolecular Elimination occurs to cleave the Ion into the products:
- DMS
- Aldehyde or Ketone depending on the Alcohol Type
What if we used a Secondary Alcohol instead of a Primary Alcohol?
SN2 Substition and Stabilization using Triethylamine

When reacting Secondary Alcohols, just add an extra R (Side Chain) in the mechanism
Ketone Formation

SN2 Substitution and Stabilization using TEA to form the Aldehyde Product
Want to try some Practice Problems?
Concept Questions

Which Alcohol Type cannot go through this reaction?
Reveal the Answer


Fill in the Needed Reagents for the Swern Oxidation Reaction
Reveal the Answer

Predict the Product

Guess the Product of this Reaction
Reveal the Answer



Predict the Product of this Reaction
Reveal the Answer

First, identify the secondary alcohol and avoid the tertiary alcohol (The OH connected with solid bonds)

The OH with the solid bonds is a tertiary alcohol which means it cannot react. The secondary alcohol below the tertiary alcohol is selectively oxidized instead


Predict the Product of this Reaction
Reveal the Answer

Primary Alcohol at the end of the chain is oxidized to an Aldehyde
Mechanism Problems

Propose a Mechanism for this Reaction
Reveal the Answer

Always Identify the Side Chains and Alcohol type to predict the correct product. Once you have done that you can proceed towards the mechanism

DMSO Resonance and Chlorosulfonium Ion Formation

Chlorodimethyl Sulfonium Ion and Alcohol Attack

SN2 Substition and Stabilization using Triethylamine and Ketone Formation
This should form the expected Ketone product as a result of selective oxidation of the secondary alcohol on this compound.
Links for Educators
Enjoy!