Oxidation with DCM
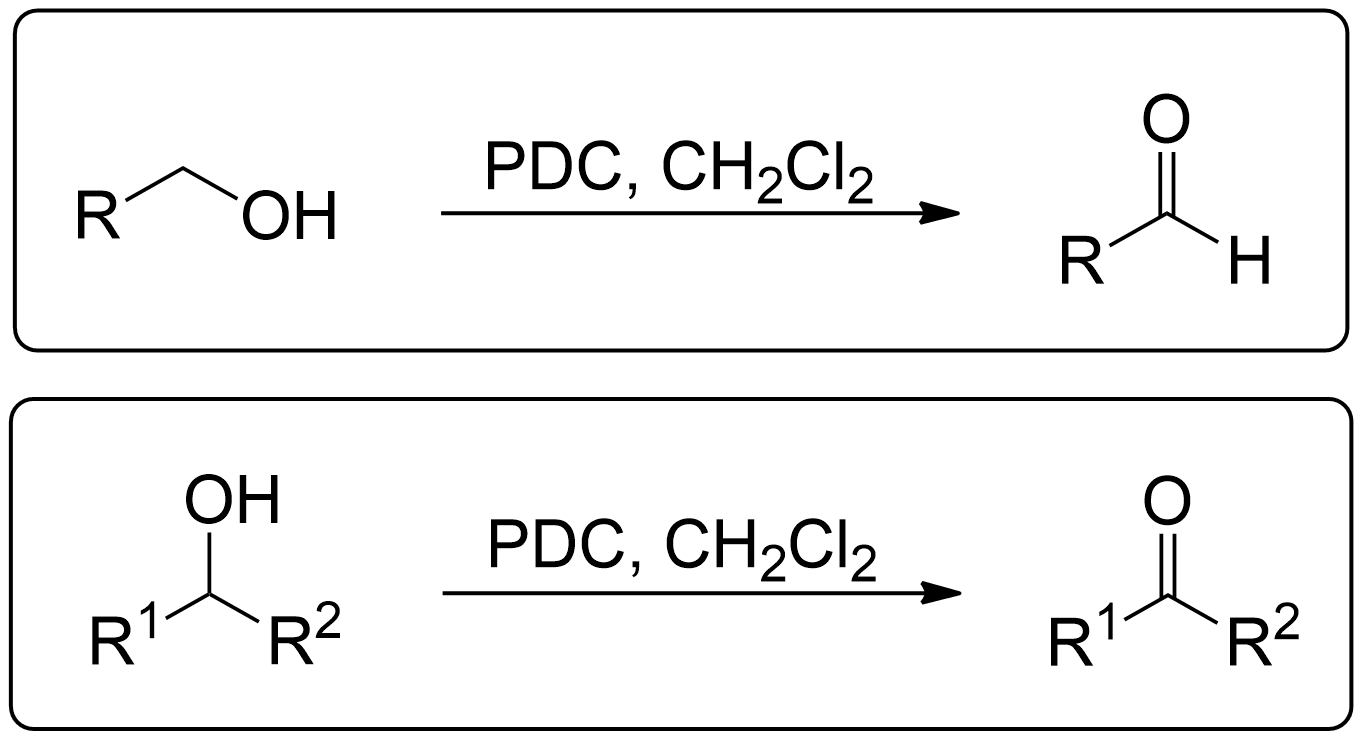
General Scheme of the Corey-Schmidt Oxidation
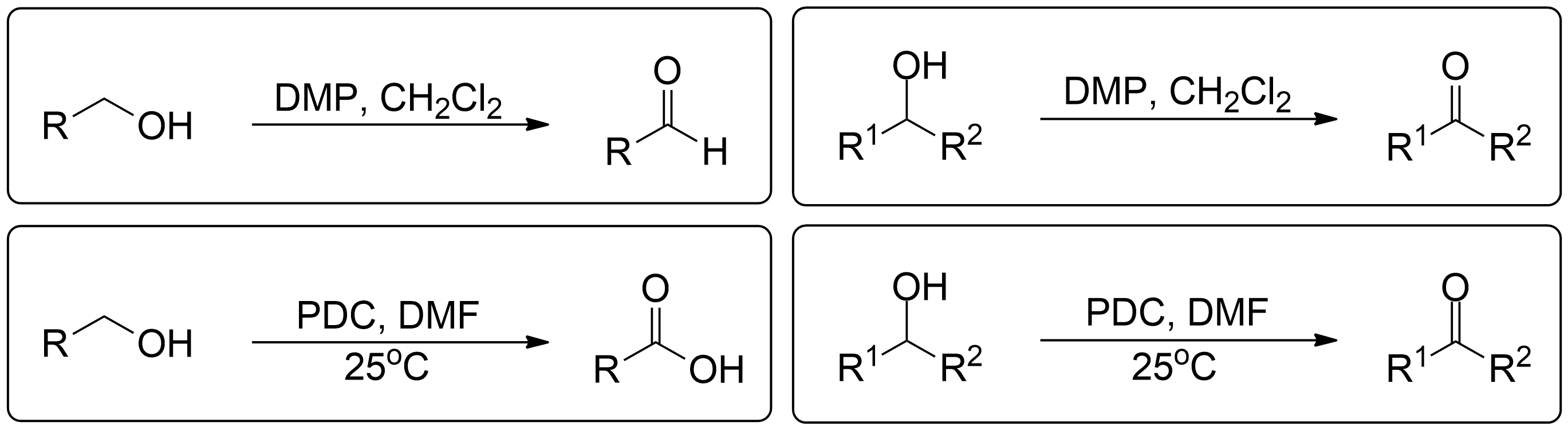
The Corey-Schmidt oxidation is a method used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones using the Pyridinium dichromate (PDC).1
Finding the Product for a 1° Alcohol
This section is a brief overview on how to find the product for a 1° Alcohol (Primary) using a example from a real scientific research paper.

Propose a Mechanism for this Reaction.
We must find out how the product was formed and the steps to form it.
Where did this Reaction come from?

Identify the Reagents
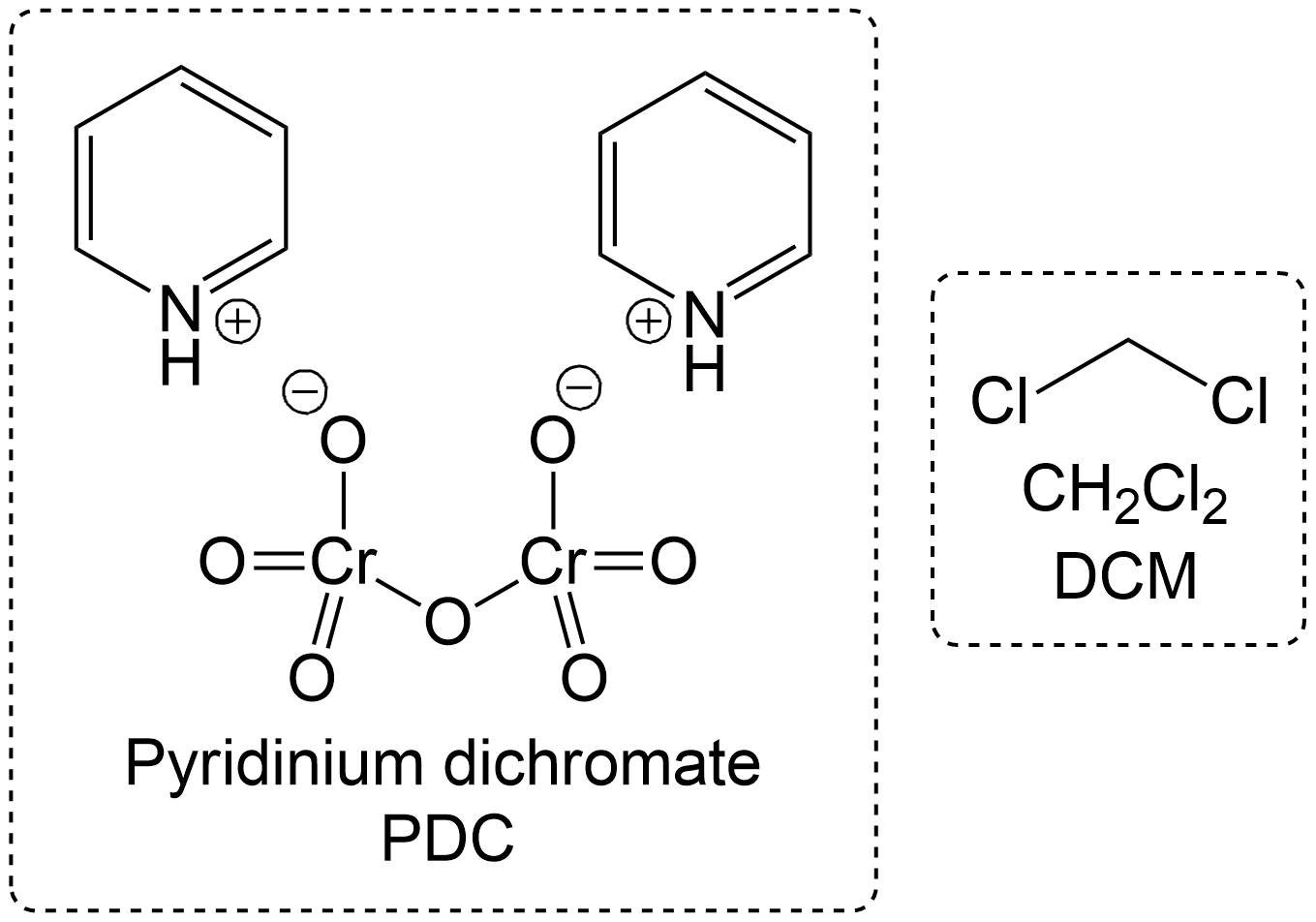
Often times, Corey Schmidt Oxidation is always performed with PDC and a solvent. In this variant, PDC is used alongside DCM, which is highly soluble in PDC. This applies to both primary and secondary alcohol oxidations.
Identify the Key Features of the Compound
Alcohol Type

These are the 3 main types of alcohols: Primary, Secondary and Tertiary.
- Primary alcohols can go through Corey-Schmidt Oxidation to become an Aldehyde.
By identifying the Alcohol Type, you now know the product to expect.
Identifying Side Chains and Alcohol Conversion

Tracking Side Chains and Alcohol Conversion
In Corey-Schmidt oxidation of primary alcohols, the process involves assigning one side chain (R) to understand the reaction better. The colored side chain represents an R group that remains unchanged during the reaction. The alcohol group is selectively oxidized to form an aldehyde. For educational purposes, we conceptually assign the non-alcohol group as R (Side chain) to visualize the changes and reconstruct the molecule post-reaction.
Guide to Side Chains
Assign the Side Chain (R): Identify the non-alcohol part of the molecule and assign it as the placeholder ‘R’ or side chain.
Understand Its Role: This placeholder helps track the unchanged part of the molecule, aiding in visualizing the structure before and after the reaction.
Focus on the Reaction Center: The primary alcohol is selectively oxidized to form an aldehyde. The placeholder shows how the structure is altered.
Reassign the Side Chain: After the reaction, reattach the placeholder R to the new aldehyde, demonstrating the unchanged nature of the side chain.
Disclaimer Warning for Writing Products

Variations on how Aldehydes may appear
They may be differently presented in different questions as shown in the image, however they are the same structure.
Mechanism for 1° Alcohol
This section is a brief overview on how to perform the mechanism for a 1° Alcohol (Primary) using the example from above.
Reactive Intermediate Formation

Formation of Reactive Intermediate
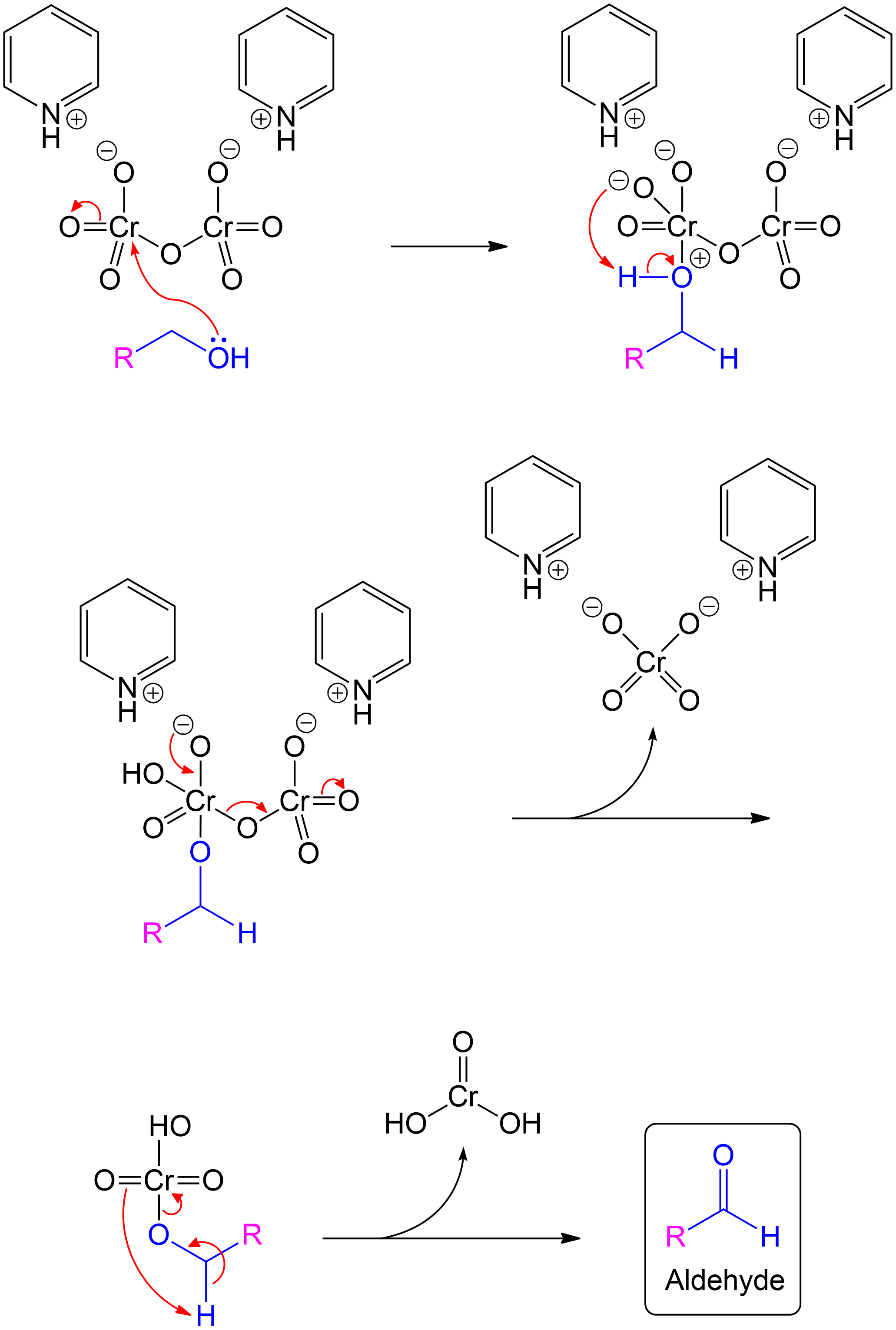
Alcohol group (-OH) performs a nucleophilic attack on the chromium center of Pyridinium dichromate molecule, forming a complex with the chromium.
In the first step of the PDC oxidation process, the alcohol group (-OH) attacks the chromium center of the PDC molecule, forming a chromium-alcohol complex. This intermediate is essential for the subsequent oxidation of the alcohol to a carbonyl compound.
Protonation of Newly Formed Intermediate

Subsequent Protonation
The newly formed intermediate is protonated to set up the intermediate for further atom rearrangement.
In this step, the newly formed chromium-alcohol complex undergoes protonation. This protonation stabilizes the intermediate, preparing it for further rearrangement and facilitating the subsequent steps in the oxidation process.
Atom Rearrangement and Elimination

Atom rearrangement and subequent elimination of chromic acid derivative
Subsequent protonation leads to atom rearrangement along with the elimination of chromic acid derivative.
Following protonation, the intermediate undergoes atom rearrangement, leading to the elimination of a chromic acid derivative. This rearrangement facilitates the formation of the final oxidized product, effectively removing the chromium species and advancing the oxidation process to completion.
Product Formation

Aldehyde product is formed after proton transfer and cleavage of oxide atom
Proton transfer and subsequent cleavage of hydrated chromium (IV) oxide yielded the desired aldehyde product
The overall products of the Corey Schmidt oxidation include the Aldehyde formed from the secondary alcohol and Chromous acid as a by-product.
Reconstructing the Target Intermediate
Finding the Product for a 2° Alcohol
This section is a brief overview on how to find the product for a 2° Alcohol (Secondary) using a example from a real scientific research paper.

Propose a Mechanism for this Reaction
Oxidation of a secondary alcohol intermediate to an ketone. The groundwork to determine the product is similar to how a primary alcohol is converted.
Where did this Reaction come from?

The two main schemes of the synthesis of (16S,20S)‐3β‐Hydroxy‐5α‐pregnane‐20,16‐carbolactam and Its N‐alkyl derivatives
Scheme one represents the synthesis of bisnorcholanic lactam derivatives via oxo‐amide intermediates. Scheme two represents the synthesis of bisnorcholanic lactam derivatives via an oxo‐acid intermediate.

This reaction showing the Corey Schmidt oxidation of the alcohol intermediate was part of scheme 1; the synthesis path leading to the formation of a bisnorcholanic lactam derivatives via oxo‐amide intermediate. Bisnorcholanic lactam derivatives, synthesized from tigogenin, are compounds featuring a cyclic amide fused with a bisnorcholanic steroid framework. These derivatives exhibit significant biological activities, including anticancer and antimicrobial properties, which is particularly valuable in medicinal applications and chemical processes.3

One key step in the synthesis involved the oxidation of a secondary alcohol intermediate (2b) to a ketone intermediate (3b) using Pyridinium dichromate (PDC) in dichloromethane (DCM) at room temperature for 4 hours. This transformation was achieved with a 62% yield and was crucial in advancing the synthesis.3
Identify the Reagents

Often times, Corey Schmidt Oxidation is always performed with PDC and a solvent. In this variant, PDC is used alongside DCM, which is highly soluble in PDC. This applies to both primary and secondary alcohol oxidations.
Identify the Key Features of the Compound
Alcohol Type

These are the 3 main types of alcohols: Primary, Secondary and Tertiary.
- Primary alcohols can go through Corey-Schmidt Oxidation to become an Aldehyde.
By identifying the Alcohol Type, you now know the product to expect.
Identifying Side Chains and Alcohol Conversion
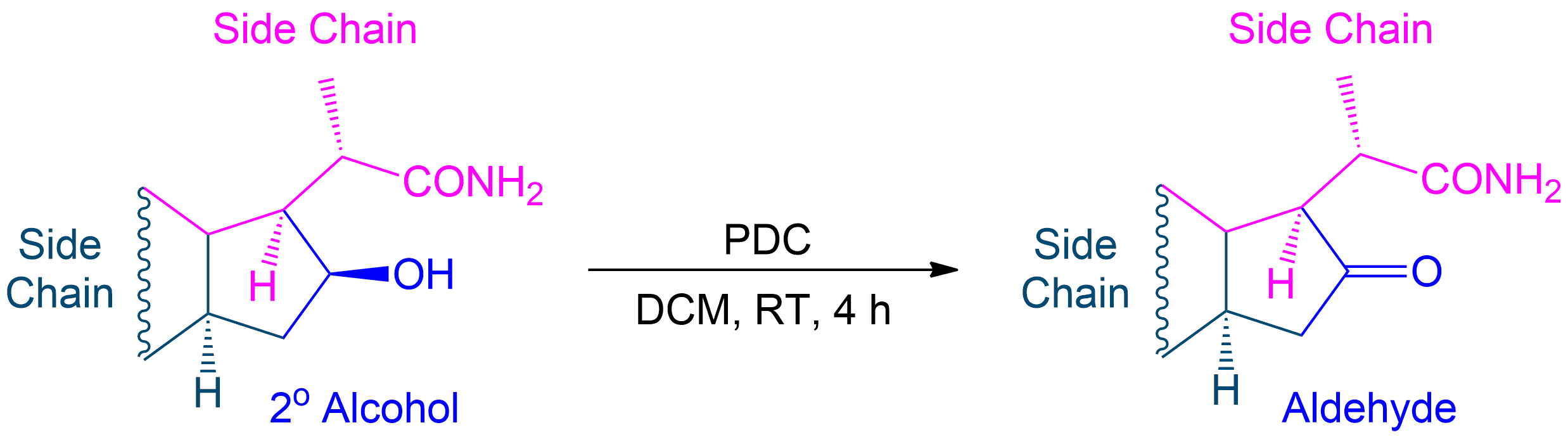
Tracking Side Chains and Alcohol Conversion in Corey Schmidt Oxidation
In Corey Schmidt oxidation of secondary alcohols, the colored side chains represent R groups that remain unchanged during the reaction. The alcohol group is selectively oxidized to form a ketone. For educational purposes,parts of the molecule are assigned and used as placeholders to demonstrate the reaction. Students! Please use parts of the molecule as placeholders: R¹ can be one part of the molecule on one side of the molecule and R² can be the remainder of the full molecule (except the secondary alcohol and the first side chain of course). This allows for easy reconstruction of the molecule after oxidation, to visually see the selective nature of the reaction.
Guide to Side Chains
Identify Side Chains (R¹ and R²): Use parts of the molecule as placeholders (R¹ and R²), representing parts of the molecule flanking the alcohol group.
Understand Their Role: These placeholders help track the unchanged parts of the molecule, aiding in visualizing the structure before and after the reaction.
Focus on the Reaction Center: The secondary alcohol is selectively oxidized to form a ketone. The placeholders show how the structure is altered.
Reassign Side Chains: After the reaction, reattach the placeholders (R¹ and R²) to the new ketone, demonstrating the unchanged nature of the side chains.
Mechanism for 2° Alcohol
This section is a brief overview on how to perform the mechanism for a 2° Alcohol (Secondary) using the example from above.
Reactive Intermediate Formation

Formation of Reactive Intermediate for a Secondary Alcohol
Alcohol group (-OH) performs a nucleophilic attack on the chromium center of Pyridinium dichromate molecule, forming a complex with the chromium.
In the first step of the PDC oxidation process, similar to the primary alcohol oxidation mechanism, the alcohol group (-OH) attacks the chromium center of the PDC molecule, forming a chromium-alcohol complex. This intermediate is essential for the subsequent oxidation of the alcohol to a carbonyl compound.
Protonation of Newly Formed Intermediate

Subsequent Protonation
The newly formed intermediate is protonated to set up the intermediate for further atom rearrangement.
In this step, the newly formed chromium-alcohol complex undergoes protonation. This step is identical to the primary alcohol oxidation steps, except an additional side chain is added. This protonation stabilizes the intermediate, preparing it for further rearrangement and facilitating the subsequent steps in the oxidation process.
Atom Rearrangement and Elimination

Atom rearrangement and subequent elimination of chromic acid derivative
Subsequent protonation leads to atom rearrangement along with the elimination of chromic acid derivative.
Following protonation, the intermediate undergoes atom rearrangement, leading to the elimination of a chromic acid derivative. This rearrangement facilitates the formation of the final oxidized product, effectively removing the chromium species and advancing the oxidation process to completion. This step is identical to the primary alcohol oxidation.
Final Overall Products

Ketone product is formed after proton transfer and cleavage of oxide atom
Proton transfer and subsequent cleavage of hydrated chromium (IV) oxide yielded the desired Ketone product
The overall products of the Corey Schmidt oxidation include the Ketone formed from the secondary alcohol and Chromous acid as a by-product.
Reconstructing the Final Overall Product
Sample Problems
Test your Knowledge.
Question 1
Predict the Product.

Reveal the Answer.
Where did this Reaction come from?

Overall Reaction used to form Mycalol (Proposed Structure) from Fragment A (6) and Fragment B (11)
The synthesis of Mycalol involved Fragments A and B (6 and 11) undergoing several other reactions to form the proposed structure of Mycalol. The proposed structure was revised based on detailed NMR analysis, changing the position of the acetate group and the stereochemistry of the glycerol unit.

This reaction showing the Corey Schmidt oxidation of this alcohol intermediate (3) was part of the proposed synthesis pathway of the formation of Mycalol, a potent anticancer agent, which was synthesized through a series of steps, notably PDC oxidation. These steps resulted in the formation of the desired aldehyde intermediate (3a), which underwent further reactions to complete the synthesis.

One main reagent used in the synthesis of Mycalol was Fragment A. Fragment A (6) was formed from the conversion process of compound 2 to compound 6 (Fragment A) over a four step synthesis pathway.
A crucial step in this multi-step process was the oxidation of a primary alcohol intermediate (3a) to an aldehyde intermediate using Pyridinium dichromate (PDC) in dichloromethane (DCM) for 4 hours. The resulting aldehyde intermediate (3a) was then further utilized in subsequent steps towards the proposed synthesis of Mycalol.2
Question 2
Propose a Mechanism for this Reaction.

Reveal the Answer.

Start by identifying the non-alcohol portion of the molecule and determine the product
Colored side chains in these diagrams represent constant R groups. Focus on the central alcohol, which transforms into a ketone or aldehyde. Use the colored chains to track and restore these groups post-reaction, highlighting the selective oxidation process. Once you have done this proceed to the mechanism.

Form the Overall Product
This should form the expected Ketone product as a result of oxidation of the secondary alcohol on this compound.
Where did this Reaction come from?

Completion of the Enantioselective total synthesis of (-)-epoxyquinols A and B
The diastereomeric (E)-dienone (-)-19 and (Z)-dienone (-)-20 were readily separated and fully characterized to form the desired product (-)-epoxyquinols A and B.
This reaction showing the Corey-Schmidt Oxidation of the alcohol intermediate (18d or 18e) to an (E)-dienone (-)-19 and (Z)-dienone (-)-20 with a selectively oxidized aldehyde group was part of the synthesis pathway of the formation of (-)-epoxyquinols A and B. (-)-epoxyquinols A and B are anti-podes of the angiogenesis inhibiting natural products which contain various biological activity profiles. These epoxyquinone natural products and their associated biological profiles, according to the authors, have sparked interest in the synthetic community.5

Mehta and Islam (2004), in this non-linear synthetic pathway, describe the successful yielding process of a epoxyquinone building block (5) derived from a Diels-Alder Adduct of cyclopentadiene and p-benzoquinone. This building block is split into two smaller pathways where they are converted to Compounds (+)-7 and (-)-7. The first pathway consisting of one step (a) Lipase PS 30 (Amano), vinyl acetate, t-butylmethylether, 0°C, 6 h, 82%; and the second pathway which consisted of two steps.
Compounds (+)-7 is subjected to a 9 step pathway which eventually yielded the (E)-dienone (-)-19 and (Z)-dienone (-)-20. They were readily separated and fully characterized to form the desired product (-)-epoxyquinols A and B.5

One key step was the oxidation of the secondary alcohol group on Compound 18d or 18e. PDC in DCM was officially used which successfully yielded the dieones needed to synthetize the epoxyquinone products.5
Summary
The reaction entry summary. Find the general scheme and full summarized mechanisms here.
General Scheme
This section briefly summarizes what can and cannot undergo reactions.

- 1° Alcohols (Primary) get oxidized to Aldehydes.
- 2° Alcohols (Secondary) get oxidized to Ketones.
- 3° Alcohols (Tertiary) do not get oxidized at all.
General Mechanism
This section briefly summarizes steps to find the product and perform the mechanisms.
Quick steps to finding the product for any alcohol
- Identify the reagents.
- Assign side chains (non alcohol part).
- Selectively convert Alcohol to correct product based on alcohol type. Nothing else.
- Keep the side chains (non alcohol part) the same and piece together the full molecule together again.
Full Primary Mechanism
Full Secondary Mechanism

Links and Related Articles
Want to Practice your knowledge or need more help? Browse related articles.
References
1. Corey, E. J.; Schmidt, G. Useful Procedures for the Oxidation of Alcohols Involving Pyridinium Dichromate in Aprotic Media. Tetrahedron Lett. 1979, 20 (5), 399–402. DOI: 10.1016/S0040-4039(01)86498-0. ↩

2. Terpstra, J. W.; Van Leusen, A. M. A new synthesis of benzo[b]thiophenes and benzo[c]thiophenes by annulation of disubstituted thiophenes. J. Org. Chem. 1986, 51 (2), 230–238. DOI: 10.1021/jo00352a019. ↩

3. Wojtkielewicz, A.; Pawelski, D.; Bazydło, P.; Baj, A.; Witkowski, S.; Morzycki, J. W. A Convenient Synthesis of (16S,20S)-3β-hydroxy-5α-pregnane-20,16-carbolactam and its N-alkyl Derivatives. Molecules 2020, 25 (10), 2377. DOI: 10.3390/molecules25102377. ↩

4. Seetharamsingh, B.; Rajamohanan, P. R.; Reddy, D. S. Total Synthesis and Structural Revision of Mycalol, an Anticancer Natural Product from the Marine Source. Org. Lett. 2015, 17 (7), 1652–1655. DOI: 10.1021/acs.orglett.5b00345. ↩

5. Mehta, G.; Islam, K. Enantioselective total synthesis of (−)-epoxyquinols A and B. Novel, convenient access to chiral epoxyquinone building blocks through enzymatic desymmetrization. Tetrahedron Lett. 2004, 45 (18), 3611–3615. DOI: 10.1016/j.tetlet.2004.03.057. ↩

