Dess-Martin Oxidation

The Dess-Martin oxidation, developed by Dr. J.C Martin and his doctoral student Daniel Benjamin Dess, is a method used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones using the Dess-Martin periodinane (DMP), a hypervalent iodine compound. This reagent is known for its mild conditions, high selectivity, and efficiency, making it a valuable tool in organic synthesis.1
Oxidation Variations
Here you can find the various types of Oxidation conditions and reagent combinations that utilize Dess Martin Oxidation.
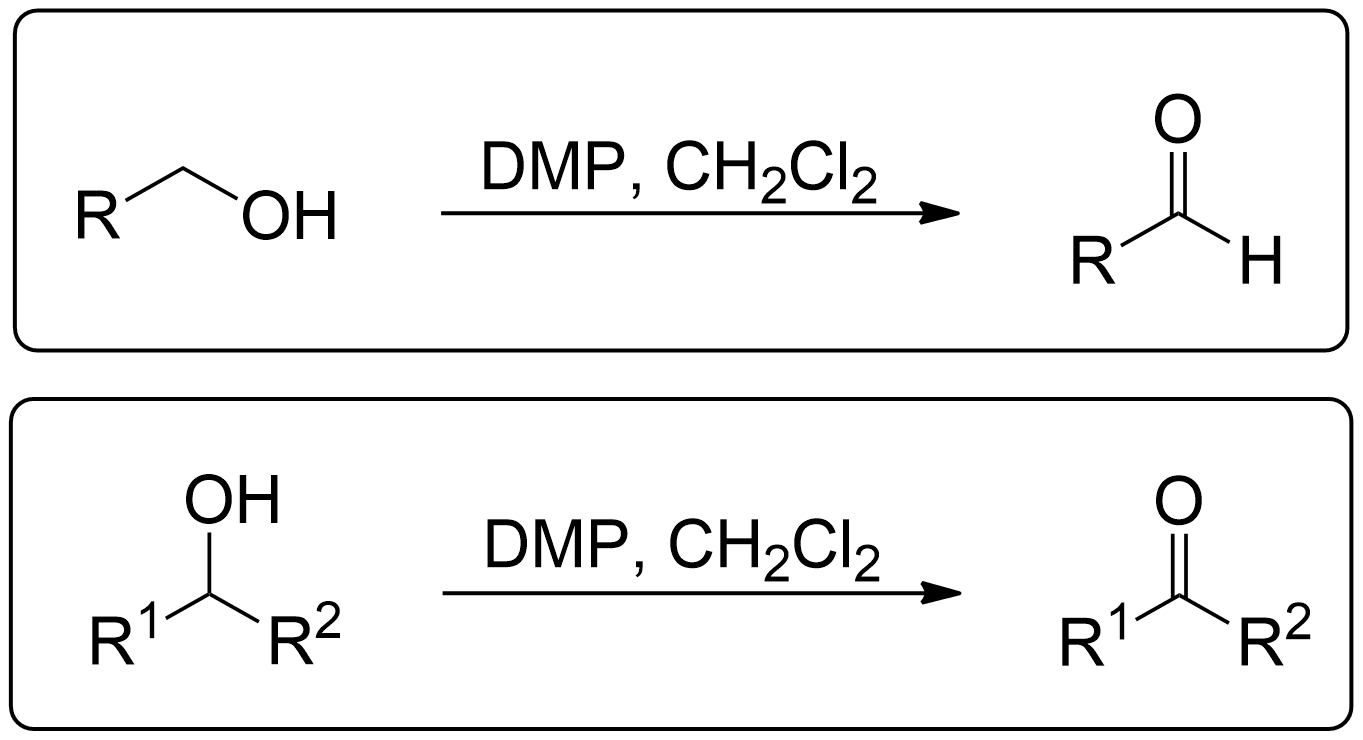
General Scheme of Dess Martin Oxidation
In the original paper "Readily Accessible 12-I-51 Oxidant for the Conversion of Primary and Secondary Alcohols to Aldehydes and Ketones." by Dess & Martin (1983), they discovered a novel and efficient reagent, periodinane 2 (DMP), used to convert primary alcohols to aldehydes and secondary alcohols to ketones.1
This was performed without the usage of water which was reported to accelerate the rate of oxidation. However, the mechanism is slightly modified in comparison the oxidation with water.

General Scheme of Dess Martin Oxidation when water is introduced.
When water is introduced, the rate of oxidation increases which accelerates the reaction. However, in comparison to the oxidation with water, the mechanism is slightly different.2

General Scheme of Dess Martin Oxidation when IBX is used.
IBX with DMSO in room temperature can be used.
In the paper "A mild oxidizing reagent for alcohols and 1,2-diols: o-iodoxybenzoic acid (IBX) in DMSO" by Frigerio & Santagostino (1994), they discovered that o-Iodoxybenzoic acid (IBX) smoothly oxidizes primary and secondary alcohols to aldehydes and ketones.3
Full Articles on the Variations
Use these to explore detailed articles on the variations.
Dess-Martin Periodinane
This section briefly goes over Dess-Martin Periodinane in detail ranging from definition to preparation.
Periodinane 2, also known as Dess-Martin Periodinane (DMP), was originally derived from 2-iodobenzoic acid and exhibited remarkable efficiency and selectivity. It was reported that DMP was soluble in other solvents such as in hexane or ether were deemed sparingly soluble. Additionally, it was deemed very soluble in dichloromethane, chloroform and acetonitrile. Dichloromethane (DCM) was reported as the primary solvent used as stated by Dess & Martin (1983).1

DMP and DCM
Dess-Martin Periodinane (DMP) has three acetoxy groups bonded to the central iodine atom. DMP is highly soluble in DCM which makes DCM, the ideal solvent to use.
Reagent Variations
This section briefly goes variations of Dess Martin Periodinane.
You may see different variations on how DMP is written out. The OAc group (Acetoxy) is a functional group you’ve seen before. The two Dess-Martin Periodinane structures depicted here are the same compound, shown in different notations and with varying ring structures.

Variations of the Dess-Martin Periodinane
Note: The Acetoxy group has been shortened. However an image reference has been provided to show what it looks like.

Acetoxy group highlighted in the full-form version of DMP alongside the hypervalent Iodine centre.
Note: The Acetoxy group has been shortened. However an image reference has been provided to show what it looks like.
Acetoxy Group vs Acetate Ion
This section briefly goes over the difference between a Acetoxy group vs Acetate ion.
Understanding the distinction between the acetate ion and the acetoxy group is crucial in grasping the chemical behavior and reactivity of molecules like DMP. While the acetoxy group is part of the structure of DMP, it becomes an acetate ion upon leaving the molecule during the oxidation reaction, playing the as role as a good leaving group to become a stable, negatively charged ion.

Differences Between Acetoxy Group and Acetate Ion
Here are the essential distinctions to understand.
| Acetoxy Group (OAc) | Acetate Ion (CH₃COO⁻) | |
|---|---|---|
| Definition | The OAc group (acetoxy) is a familiar functional group consisting of an acetyl group (CH₃CO) bonded to an oxygen atom. | The acetate ion is a charged particle with a negative electrical charge, formed when an acetoxy group is expelled and loses a hydrogen atom. |
| Role in DMP | In DMP, three acetoxy groups are bonded to the central iodine atom, making them part of the larger molecule. | Has no role in DMP itself. |
| Formation & Notation | R-OAc, where R represents the rest of the DMP molecule. | In reactions, when the OAc group is expelled from DMP, it becomes an acetate ion (CH₃COO⁻), carrying a negative charge as it takes an extra electron pair. |
Reagent Preparation
This section briefly explains how to prepare Dess Martin Periodinane.

Creation of DMP in a 2 step synthesis.
Note: Dess-Martin Periodinane is written in with in it’s shortform.
The preparation of Dess-Martin Periodinane (DMP) involves two key steps:
Initial Treatment
First, 2-iodobenzoic acid is treated with potassium bromate (KBrO3) in sulfuric acid (H2SO4), maintaining the temperature below 55°C during the addition of KBrO3. The mixture is then warmed to 65°C and stirred for 3.6 hours, producing the cyclic tautomer of 2-iodoxybenzoic acid with a 93% yield.1
Product Formation
Next, a stirred slurry of 2-iodoxybenzoic acid in acetic anhydride and acetic acid is heated to 100°C until the solid dissolves. The solvent is then removed under vacuum at room temperature, resulting in a thick slurry, which is filtered in an inert atmosphere and washed with ether, yielding Periodinane 2 with an 87% overall yield from the iodobenzoic acid.1
Reagent Facts
This section goes over some universally known facts retrieved from the original paper by Dess & Martin (1983).
DMP is easy to prepare, remains stable under proper storage conditions and is environmentally friendly, producing benign by-products compared to toxic chromium-based oxidizers which often required time-consuming filitration procedures.1
Reagent Resources
This section displays different databases that host more information on Dess Martin Periodinane.
Chemspider
Pubchem
Tribute to Dr. James Cullen Martin
This section is a tribute to the late Dr. James Cullen Martin (1928-1999).

James Cullen Martin (1928-1999), a prominent figure in organic chemistry community known for his work on hypervalent compounds, passed away on April 20, 1999. Martin's most notable contribution is the invention of the Dess-Martin Periodinane (DMP) in 1983, developed with his doctoral student Daniel Benjamin Dess.4
This reagent revolutionized the oxidation of alcohols, offering a straightforward synthesis and mild reaction conditions. DMP efficiently oxidizes primary alcohols to aldehydes and secondary alcohols to ketones, avoiding toxic by-products and simplifying the process. Its selective and environmentally friendly properties have made it a staple in laboratories worldwide.
Rest in Peace,
Dr. J.C. Martin
Related Articles
Browse all related Articles.
References

1. Dess, D. B.; Martin, J. C. Readily Accessible 12-I-51 Oxidant for the Conversion of Primary and Secondary Alcohols to Aldehydes and Ketones. J. Org. Chem. 1983, 48, 4155–4156. DOI: 10.1021/jo00356a052. ↩

2. Meyer, S. D.; Schreiber, S. L. Acceleration of the Dess-Martin Oxidation by Water. J. Org. Chem. 1994, 59, 7549–7552. DOI: 10.1021/jo00103a067. ↩

3. Frigerio, M.; Santagostino, M. A mild oxidizing reagent for alcohols and 1,2-diols: o-iodoxybenzoic acid (IBX) in DMSO. Tetrahedron Lett. 1994, 35, 8019–8022. DOI: 10.1016/0040-4039(94)80038-3. ↩
4. University of Illinois Archives, "Martin, James C." Available at: https://archives.library.illinois.edu/archon/?p=creators/creator&id=3886. Accessed June 29, 2024. ↩
5. Akiba, K.-y. Memoirs of Professor James Cullen Martin. Phosphorus, Sulfur, and Silicon 2006, 181, 1201–1215. DOI: 10.1080/10426500500326321. ↩


