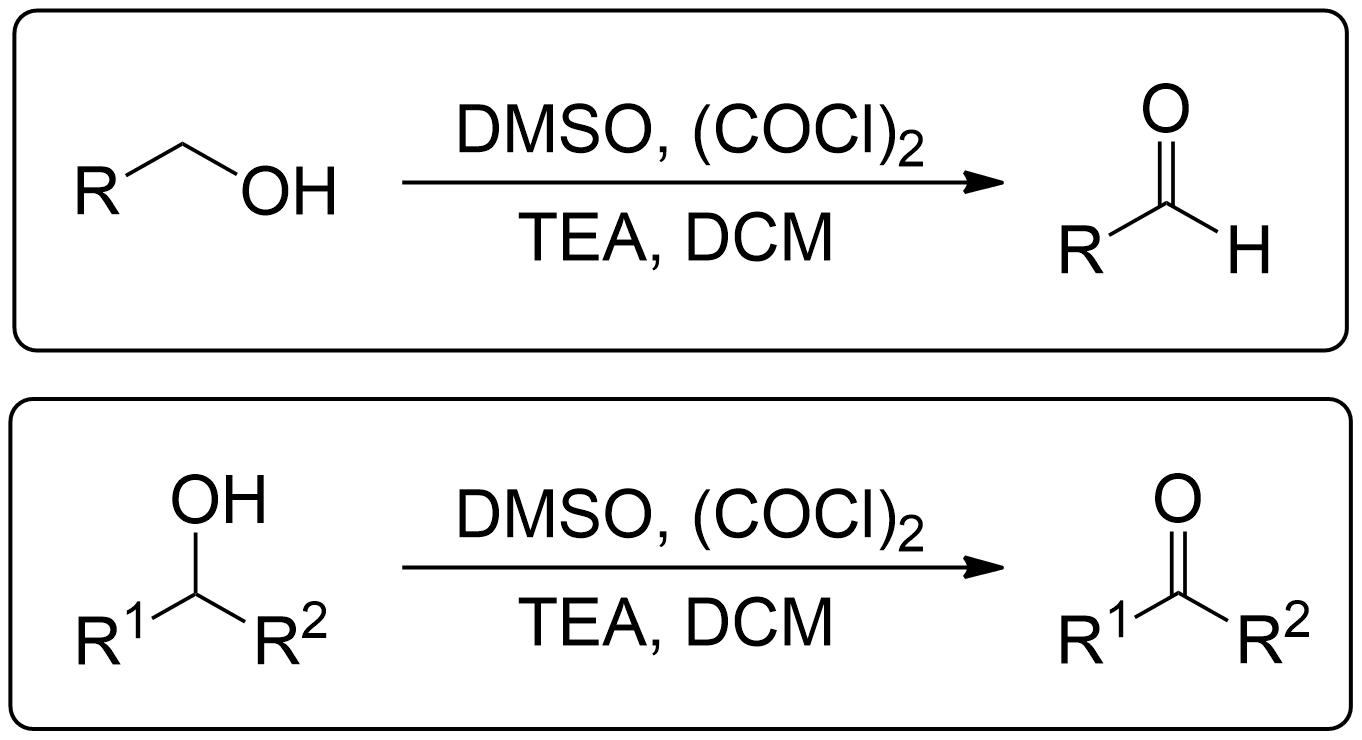
Oxidation with Oxalyl chloride
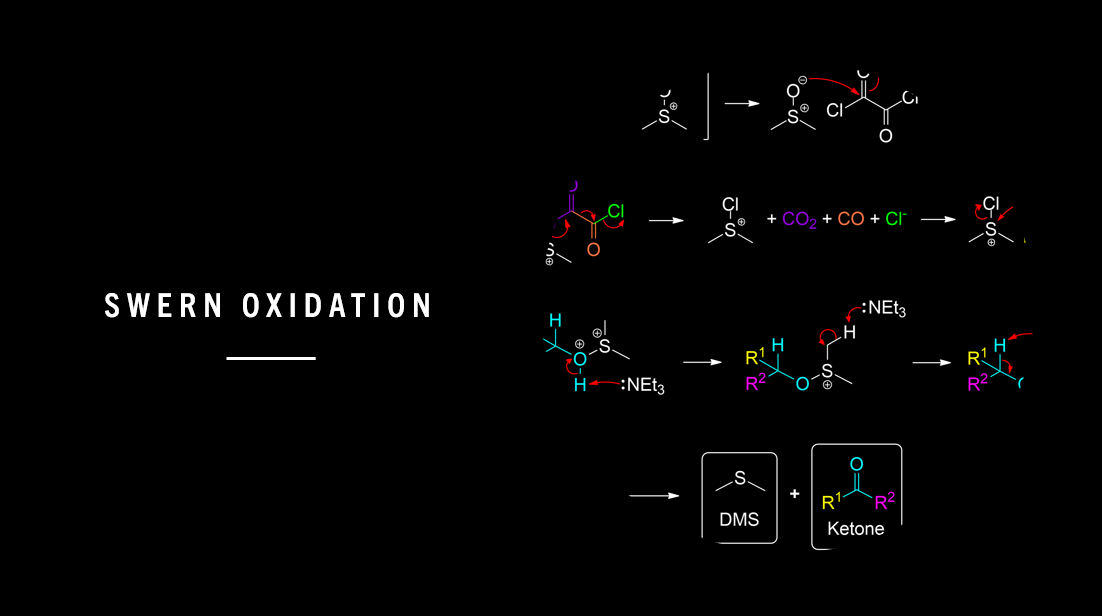
General Scheme of the Swern Oxidation
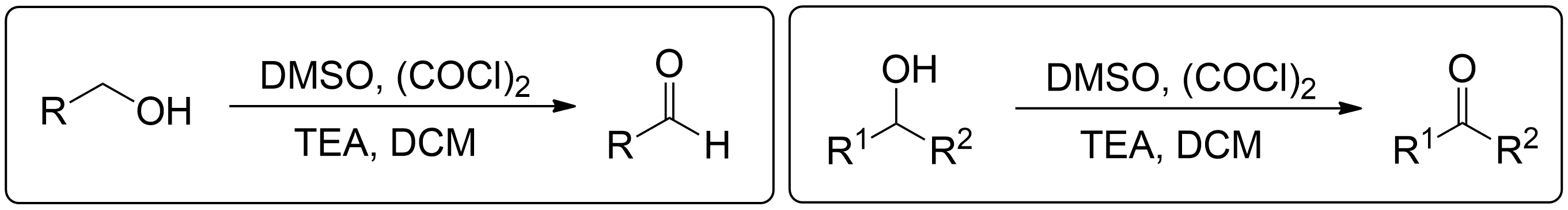
The Swern oxidation, developed by Kanji Omura and Daniel Swern in 1978, is a method used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones using activated dimethyl sulfoxide (DMSO), oxalyl chloride as a oxidizing agent, triethylamine (TEA) and dichloromethane (DCM).1
DMSO and Oxalyl chloride form a reactive salt (Lewis Acid) with the alcohol providing a good leaving group required for subsequent elimination.
Finding the Product for a 1° Alcohol
This section is a brief overview on how to find the product for a 1° Alcohol (Primary) using a example from a real scientific research paper.

Propose a Mechanism for this Reaction.
We must find out how the product was formed and the steps to form it.
Where did this Reaction come from?

Condensed Synthesis Overview of the Asymmetric Total Synthesis of Taxol by Mukaiyama et al. (1990)
This 61 step linear synthesis features Swern Oxidation for a total of 4 steps out of 61 steps. L-serine is the starting material and undergoes 61 stpes to form Taxol.

In 1999, Mukaiyama et al. published the Mukaiyama Asymmetric Total Synthesis of Taxol. Taxol, a well-known complex organic molecule, underwent retrosynthetic analysis, revealing an optically active ketone intermediate (3), which could be further simplified into a chiral aldehyde intermediate (4).

As seen in scheme 4, the chiral aldehyde intermediate (4), compound 18 undergoes a reduction via DIBAL and hexane as a solvent. Next, this reduced intermediate undergoes Swern Oxidation to form the chiral aldehyde intermediate (4).2
Identify the Reagents
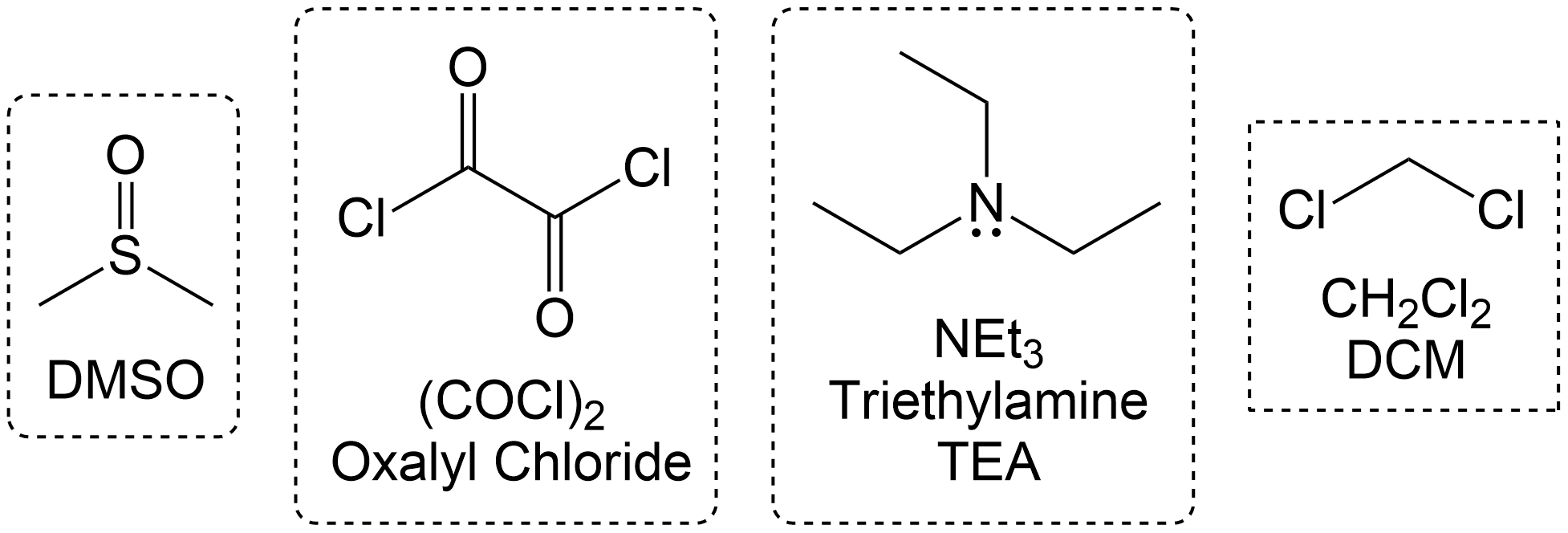
DMSO is used alongside the preferred oxidizing agent Oxalyl chloride, TEA (Triethylamine) and DCM.
Identify the Key Features of the Compound
Alcohol Type

These are the 3 main types of alcohols: Primary, Secondary and Tertiary.
- Primary alcohols can go through Swern Oxidation to become an Aldehyde.
By identifying the Alcohol Type, you now know the product to expect.
Identifying Side Chains and Alcohol Conversion

Tracking Side Chains and Alcohol Conversion
In the Swern oxidation of primary alcohols, the process involves assigning one side chain (R) to understand the reaction better. The colored side chain represents an R group that remains unchanged during the reaction. The alcohol group is selectively oxidized to form an aldehyde. For educational purposes, we conceptually assign the non-alcohol group as R (Side chain) to visualize the changes and reconstruct the molecule post-reaction.
Guide to Side Chains
Assign the Side Chain (R): Identify the non-alcohol part of the molecule and assign it as the placeholder ‘R’ or side chain.
Understand Its Role: This placeholder helps track the unchanged part of the molecule, aiding in visualizing the structure before and after the reaction.
Focus on the Reaction Center: The primary alcohol is selectively oxidized to form an aldehyde. The placeholder shows how the structure is altered.
Reassign the Side Chain: After the reaction, reattach the placeholder R to the new aldehyde, demonstrating the unchanged nature of the side chain.
Disclaimer Warning for Writing Products

Variations on how Aldehydes may appear
They may be differently presented in different questions as shown in the image, however they are the same structure.
Mechanism for 1° Alcohol
This section is a brief overview on how to perform the mechanism for a 1° Alcohol (Primary) using the example from above.
DMSO undergoes Resonance

Resonance forms of DMSO
DMSO is capable of undergoing resonance. This is important for the next step.
In the first step of Swern Oxidation, DMSO undergoes resonance to prepare the DMSO to perform a nucleophilic attack on Oxalyl chloride.
Chlorosulfonium Ion Formation

Nucleophillic attack using DMSO Resonance structure
DMSO Resonance Structure performs Nucleophilic Attack, Chloride Ion acts as a Leaving Group.
In this step, the newly formed chromium-alcohol complex undergoes protonation. This protonation stabilizes the intermediate, preparing it for further rearrangement and facilitating the subsequent steps in the oxidation process.
Chlorodimethyl Sulfonium Ion and Byproduct Formation

Chlorodimethyl Sulfonium Ion Formation
The nucleophilic attack initiates proton transfer within the Chlorosulfonium Ion to form Chlorodimethyl Sulfonium Ion its Byproduct.
In this step, the Chlorosulfonium Ion decomposes after proton transfer is initiated from the chloride ion. This action releases carbon dioxide, carbon monoxide and a chloride ion.
Alcohol and Base Addition

Addition of the Primary Alcohol and 2 equivalents of TEA (Triethylamine).
This process produces a alkoxysulfonium ion intermediate. However the octet rule is violated in the newly produced ion, so TEA (Triethylamine) is needed to stabilize the molecule for further transformation.
In this step, the primary alcohol is added and SN2 substitution occurs. Chloride is a good leaving group and leaves the Chlorosulfonium Ion to produce alkoxysulfonium ion intermediate. Next, 2 equivalents of TEA (Triethylamine) is added to neutralize changes and stabilize the intermediate. This eventually forms a sulfur ylide.
Ylide Formation and Intramolecular Elimination

Aldehyde product and DMS byproduct Formation
Sulfur Ylide decomposes to form DMS and the desired aldehyde product.
The Sulfur ylide undergoes intramolecular elimination to cleave the ylide into the desired product and by-products.
Reconstructing the Target Intermediate
Finding the Product for a 2° Alcohol
This section is a brief overview on how to find the product for a 2° Alcohol (Secondary) using a example from a real scientific research paper.

Propose a Mechanism for this Reaction
Oxidation of a secondary alcohol intermediate to an ketone. The groundwork to determine the product is similar to how a primary alcohol is converted.
Where did this Reaction come from?
Identify the Reagents

Often times, Swern Oxidation with oxalyl chloride is performed alongside TEA (Triethylamine) and
Identify the Key Features of the Compound
Alcohol Type

These are the 3 main types of alcohols: Primary, Secondary and Tertiary.
- Primary alcohols can go through Swern Oxidation to become an Aldehyde.
By identifying the Alcohol Type, you now know the product to expect.
Identifying Side Chains and Alcohol Conversion

Tracking Side Chains and Alcohol Conversion in Swern Oxidation
Firstly, find the alcohol structure and highlight it. Next, assign side chains (R groups) that are on the opposite sides of the alcohol group.
When oxidizing secondary alcohols, the colored side chains represent unchanged R groups. The alcohol is selectively oxidized to form a ketone. For educational purposes, use R¹ and R² as placeholders for parts of the molecule, excluding the secondary alcohol and first side chain. This helps visualize the reaction and reconstruct the molecule post-oxidation.
Guide to Side Chains
Identify Side Chains (R¹ and R²): Use parts of the molecule as placeholders (R¹ and R²), representing parts of the molecule flanking the alcohol group.
Understand Their Role: These placeholders help track the unchanged parts of the molecule, aiding in visualizing the structure before and after the reaction.
Focus on the Reaction Center: The secondary alcohol is selectively oxidized to form a ketone. The placeholders show how the structure is altered.
Reassign Side Chains: After the reaction, reattach the placeholders (R¹ and R²) to the new ketone, demonstrating the unchanged nature of the side chains.
Mechanism for 2° Alcohol
This section is a brief overview on how to perform the mechanism for a 2° Alcohol (Secondary) using the example from above.
DMSO undergoes Resonance

Resonance forms of DMSO
DMSO is capable of undergoing resonance. This is important for the next step.
In the first step of Swern Oxidation, DMSO undergoes resonance to prepare the DMSO to perform a nucleophilic attack on Oxalyl chloride.
Chlorosulfonium Ion Formation

Nucleophillic attack using DMSO Resonance structure
DMSO Resonance Structure performs Nucleophilic Attack, Chloride Ion acts as a Leaving Group.
In this step, the newly formed chromium-alcohol complex undergoes protonation. This protonation stabilizes the intermediate, preparing it for further rearrangement and facilitating the subsequent steps in the oxidation process.
Chlorodimethyl Sulfonium Ion and Byproduct Formation

Chlorodimethyl Sulfonium Ion Formation
The nucleophilic attack initiates proton transfer within the Chlorosulfonium Ion to form Chlorodimethyl Sulfonium Ion its Byproduct.
In this step, the Chlorosulfonium Ion decomposes after proton transfer is initiated from the chloride ion. This action releases carbon dioxide, carbon monoxide and a chloride ion.
Alcohol and Base Addition

Addition of the Secondary Alcohol and 2 equivalents of TEA (Triethylamine).
This process produces a alkoxysulfonium ion intermediate. However the octet rule is violated in the newly produced ion, so TEA (Triethylamine) is needed to stabilize the molecule for further transformation. This process is the same as the primary alcohol, except there is an additional side chain.
In this step, the primary alcohol is added and SN2 substitution occurs. Chloride is a good leaving group and leaves the Chlorosulfonium Ion to produce alkoxysulfonium ion intermediate. Next, 2 equivalents of TEA (Triethylamine) is added to neutralize changes and stabilize the intermediate. This eventually forms a sulfur ylide.
Ylide Formation and Intramolecular Elimination

Ketone product and DMS byproduct Formation
Sulfur Ylide decomposes to form DMS and the desired aldehyde product.
The Sulfur ylide undergoes intramolecular elimination to cleave the ylide into the desired product and by-products.
Reconstructing the Final Overall Product
Sample Problems
Test your Knowledge.
Question 1
Predict the Product.

Reveal the Answer.
Where did this Reaction come from?

Overall Synthesis of (+)-Pentacycloanammoxic Acid from the starting material cyclooctatetraene
Cyclooctatetraene underwent a 15 step synthesis pathway to form the end product:(+)-Pentacycloanammoxic Acid.

The starting material, Cyclooctatetraene underwent 10 steps until it reached the formation of compound 7. Compound 7 underwent subsequent reactions, including reduction from DIBAL-H and oxidation via Swern Oxidation to form compound 8.
Question 2
Propose a Mechanism for this Reaction.

Reveal the Answer.
Determine Side Chains and Alcohol Conversion

Tracking Side Chains and Alcohol Conversion in Swern Oxidation
Be careful when assigning side chain placeholders. If you do not see a side chain visible that is not denoted by H or another group. Assume its a Methyl group. In the example, it was not shown, however for group tracking we have shown it in red.
When oxidizing secondary alcohols, the colored side chains represent unchanged R groups. The alcohol is selectively oxidized to form a ketone. For educational purposes, use R¹ and R² as placeholders for parts of the molecule, excluding the secondary alcohol and first side chain. This helps visualize the reaction and reconstruct the molecule post-oxidation.
Perform the Mechanism

Reconstruct the final molecule
Where did this Reaction come from?

Condensed Synthesis Overview of the Asymmetric Total Synthesis of Taxol by Mukaiyama et al. (1990)
This 61 step linear synthesis features Swern Oxidation for a total of 4 steps out of 61 steps.

The Mukaiyama Asymmetric Total Synthesis of Taxol was published in 1999 by Mukaiyama et al (1999). Taxol, a well known and difficult complex organic molecule, underwent retrosynthetic analysis. This revealed a optically active ketone intermediate (3) which could be further broken down into another basic unit (4)

As seen in Scheme 5 of the paper, the synthesis of an optically active ketone (3) involved a single step process using two sets of reagent combinations. First, the alkylation (Grignard reaction) of compound 22 using Methyl Magnesium Bromide (MeMgBr) This yielded an alcohol intermediate.2
Next, this secondary alcohol intermediate was oxidized to a ketone (3) using Swern Oxidation (Oxidation with Oxalyl chloride).2
Summary
The reaction entry summary. Find the general scheme and full summarized mechanisms here.
General Scheme
This section briefly summarizes what can and cannot undergo reactions.

- 1° Alcohols (Primary) get oxidized to Aldehydes.
- 2° Alcohols (Secondary) get oxidized to Ketones.
- 3° Alcohols (Tertiary) do not get oxidized at all.
General Mechanism
This section briefly summarizes steps to find the product and perform the mechanisms.
Quick steps to finding the product for any alcohol
- Identify the reagents.
- Assign side chains (non alcohol part).
- Selectively convert Alcohol to correct product based on alcohol type. Nothing else.
- Keep the side chains (non alcohol part) the same and piece together the full molecule together again.
Full Primary Mechanism

Full Secondary Mechanism

Links and Related Articles
Browse all related Articles.
References
1. Omura, K.; Swern, D. Oxidation of Alcohols by “Activated” Dimethyl Sulfoxide. A Preparative, Steric and Mechanistic Study. Tetrahedron 1978, 34 (11), 1651–1660. DOI: 10.1016/0040-4020(78)80197-5. ↩

2. Mukaiyama, T.; Shiina, I.; Iwadare, H.; Saitoh, M.; Nishimura, T.; Ohkawa, N.; Sakoh, H.; Nishimura, K.; Tani, Y.-i.; Hasegawa, M.; Yamada, K.; Saitoh, K. Asymmetric Total Synthesis of Taxol. Chem. Eur. J. 1999, 5 (1), 121–161. DOI: 10.1002/(SICI)1521-3765(19990104)5:1<121::AID-CHEM121>3.0.CO;2-O. ↩

3. Mascitti, V.; Corey, E. J. Total Synthesis of (±)-Pentacycloanammoxic Acid. J. Am. Chem. Soc. 2004, 126 (48), 15664–15665. DOI: 10.1021/ja044089a. ↩