Oxidation with TFAA
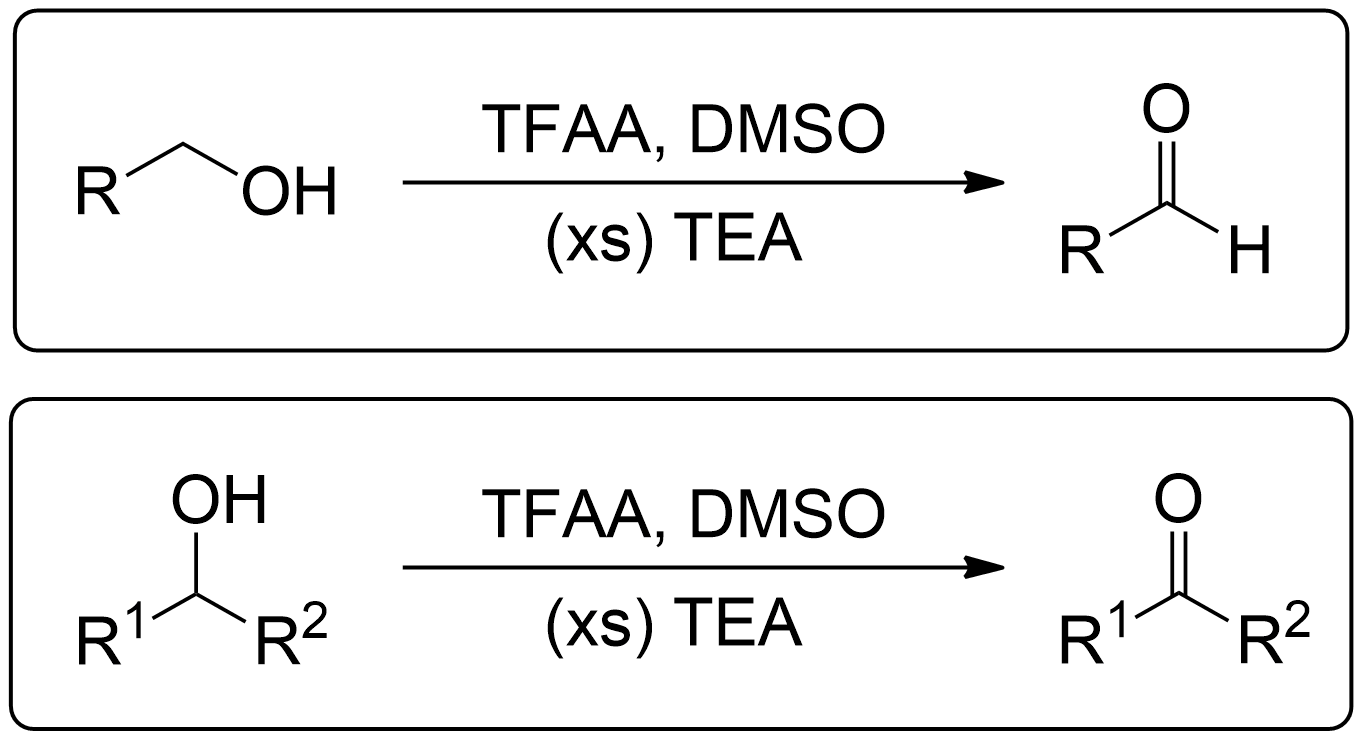
General Scheme of Swern Oxidation using TFAA
Swern Oxidation, a mild oxidation method used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones is able to use TFAA as an activator of DMSO. This method is costly and less effective than Oxalyl chloride but was a effective activator prior to the usage of oxalyl chloride.1
Summary
The reaction entry summary. Find the general scheme and full summarized mechanisms here.
General Scheme
This section briefly summarizes what can and cannot undergo reactions.

- 1° Alcohols (Primary) get oxidized to Aldehydes.
- 2° Alcohols (Secondary) get oxidized to Ketones.
- 3° Alcohols (Tertiary) do not get oxidized at all.
General Mechanism
This section briefly summarizes steps to find the product and perform the mechanisms.
Quick steps to finding the product for any alcohol
- Identify the reagents.
- Assign side chains (non alcohol part).
- Selectively convert Alcohol to correct product based on alcohol type. Nothing else.
- Keep the side chains (non alcohol part) the same and piece together the full molecule together again.
Full Primary Alcohol to Aldehyde Mechanism

Full Secondary Alcohol to Ketone Mechanism

References
1. Omura, K.; Swern, D. Oxidation of Alcohols by “Activated” Dimethyl Sulfoxide. A Preparative, Steric and Mechanistic Study. Tetrahedron 1978, 34 (11), 1651–1660. DOI: 10.1016/0040-4020(78)80197-5. ↩